Prof. Dr. rer. nat. Ulrike Stein

Experimental and Clinical Research Center,
Charité - Universitätsmedizin Berlin and
Max-Delbrück Center for Molecular Medicine
Head: Translational Oncology of Solid Tumors
Robert-Rössle-Str. 10
13092 Berlin
Tel: +49 30 9406 343
Fax: +49 30 9406 2780
E-Mail: ustein(at)mdc-berlin.de
https://www.mdc-berlin.de/stein
University Education
- 1979-1984 Martin-Luther University Halle, Diploma in Biochemstry
- 1987-1991 Academy for Clinical Education, Berlin, Graduation in Medicinal Biochemistry
Professional Experience (selection)
- 1984-1991 PhD, Humboldt-University Berlin
- 1991-1993 Post-Doc MDC Berlin
- 1994-1995 Post-Doc National Cancer Institute, Frederick, MD, USA (Alumni one month research stays 1996, 1997, 1998, 2001, 2003, 2007, 2011)
- 1996-2000 Group leader MDC
- 2001-2006 Group leader Robert-Rössle-Clinic, Berlin
- 2003 Habilitation, Charité University Medicine, Berlin
- 2007- Group leader ECRC, Berlin
- 2009- Professorship, Charité University Medicine, Berlin
- Since 2016 Elected Board member, Metastasis Research Society, Deputy of Europe
Most important Awards, Grants or Scientific Achievements
- 1994-1995 Feodor-Lynen-fellowship, Alexander von Humboldt foundation / Fogarty International Center, Bethesda, MD, USA
- 2005 Award, Epithelial Mesenchymal Transition Conference, Vancouver, BC, Canada
- 2009 Wissenschaftspreis (preclinical/translational part), AIO of the German Cancer Society
- 2010 Award of the Berlin-Brandenburg Academy of Science, Monika Kutzner foundation
- 2011 Award, 16. World Congress on Advances in Oncology, Rhode, Greece
- 2014 Felix-Burda-Award, Berlin
Research Fields
- i) Molecular and Translational Oncology
- ii) Metastasis and Biomarkers: Prognosis and Prediction
- iii) Chemoprevention and Therapeutic Intervention of Tumor Progression and Metastasis
Scientific Scope
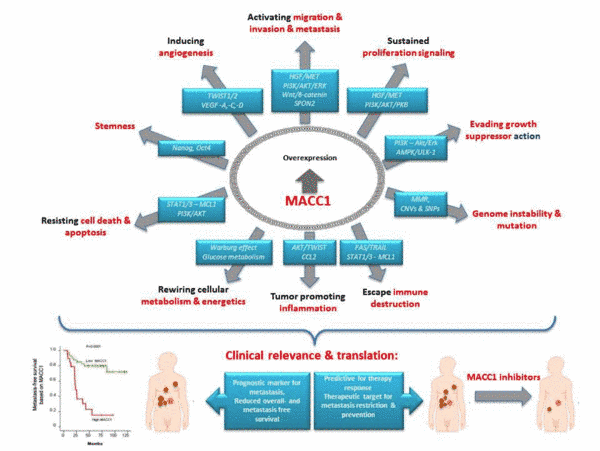
Cancer metastasis is responsible for more than 90% of cancer deaths and remains a major treatment challenge. Our translational concepts aim at identification and exploitation of novel key molecules in metastasis for prognosis and therapy of solid cancers. Our group is devoted to thorough analysis of key drivers of metastasis, their transcriptional and functional regulation, their role in cancer cell signaling and functional impact in metastasis formation. In search of new metastasis drivers we identified the novel, previously undescribed gene Metastasis-Associated in Colon Cancer 1 (MACC1) in human colorectal cancer (CRC). MACC1 induces fundamental processes like proliferation, migration, invasiveness and metastasis in xenografted and transgenic mice. Meanwhile, MACC1 has been established by us and many other groups as key player, prognostic and predictive biomarker for tumor progression and metastasis in more than 20 solid cancer entities. Following our initial discovery of MACC1, about 240 articles (PubMed) about MACC1 were published until today from groups worldwide, incl. meta-analyses on solid cancers, HCC, CRC, gastric cancer and digestive system. In recent years, we unveiled the transcriptional, post-transcriptional and post-translational regulation of MACC1. We discovered transcriptional targets and protein-protein interactors, and unveiled them as new diagnostic, prognostic and predictive key players for tumor progression and metastasis. We generated the first transgenic MACC1 mouse models, discovering MACC1-induced cancer transition and its link to stemness. We identified the first transcriptional and post-translational small molecule inhibitors, restricting MACC1-induced metastasis formation in mice. Together with the metastasis inducer S100A4, which we first identified as Wnt-signaling target gene, we generalized the impact of these biomarkers for early identification of high risk cancer patients. We exploited this knowledge for improved prognosis and response prediction using tumors and blood of solid cancer patients. Novel therapeutic approaches are currently tested in clinical trials to treat patients with metastatic disease using newly identified repositioned small molecule inhibitors acting on these biomarkers.
Future directions
1.) Cancer cell signaling of metastasis inducing genes, their networks and axes
- Identification of the MACC1 role in cancer cell signaling with focus on transcriptional targets, their regulation and the MACC1-centered signalosome
- MACC1 impact on cancer invasion and migration by single cell sequencing
- Finding transcriptional inhibitors for MACC1 for inhibition of cancer progression and metastasis.
- Tighten the molecular network around MACC1 in metastasis formation and searching for novel therapeutic intervention points
- Impact of MACC1 on cancer cell resistance and overcoming strategies for improved therapy
2.) Preclinical models for understanding and intervening in tumor progression and metastasis
- Analysis of physiological function of MACC1 with emphasis on embryogenesis/development using KO mouse models
- Evaluation of predictive biomarkers from pre-clinical cancer models for personalized anti-metastatic therapies
- Generation and analysis of novel genetically modified mouse models
3.) Molecularly targeted intervention strategies for restriction and prevention of cancer metastasis
- Development of new drugs targeting our identified biomarkers
- Development of novel anti-metastatic drugs
- Evaluation of suitable combination therapies against metastasis with novel and/or approved and repositioned drugs
4.) Clinical translation for improved prognosis, prediction and restriction of tumor progression and metastasis
- Clinical trials for single and combinatorial targeted intervention of metastasis in biomarker screened cancer patients
Group members
Selected References
- Gohlke, B.-O., Zincke, F., Eckert, A., Kobelt, D., Preissner, S., Liebeskind, J.M., Gunkel, N., Putzker, K., Lewis, J., Arbach, O., Preissner, S., Kortüm, B., Walther, W., Mura, C., Bourne, P.E., Stein, U.*, Preissner, R.* (2022) "Real-world evidence for preventive effects of statins on cancer incidence: a trans-atlantic analysis." Clin Transl Med, 12:e726.
- Dahlmann, M., Gambara, G., Brzezicha, B., Popp, O., Pachmayr, E., Gül-Klein, S., Brandl, A., Schweiger-Eisbacher, C., Mertins, P., Hoffmann, J.*, Keilholz, U.*, Walther, W.*, Regenbrecht, C.*, Rau, B.*, Stein, U.* (2021) "Peritoneal metastasis of colorectal cancer (pmCRC): Identification of predictive molecular signatures by a novel preclinical platform of matching pmCRC PDX/PD3D models." Mol Cancer, 20:129.
- Budczies, K., Kluck, K., Walther, W., Stein, U. (2020) "Decoding and targeting the molecular basis of MACC1-driven metastatic spread: lessons from big data mining and clinical-experimental approaches." Seminars Cancer Biol 60:365-379.
- Rohr, U.P., Herrmann, P., Ilm, K., Zhang, H., Lohmann, S., Reiser, A., Muranyi, A., Smith, J., Burock, S., Osterland, M., Leith, K., Singh, S., Brunhoeber, P., Bowermaster, R., Tie, J., Christie, M., Wong, H.L., Waring, P., Shanmugam, K., Gibbs, P., Stein, U. (2017) "Prognostic value of MACC1 and proficient mismatch repair status for recurrence risk prediction in stage II colon cancer patients: the BIOGRID studies." Ann Oncol 28:1869-1875.
- Stein, U., Walther, W., Arlt, F., Schwabe, H., Smith, J., Fichtner, I., Birchmeier, W., Schlag, P.M. (2009) "MACC1, a newly identified key regulator of HGF/Met signaling, predicts colon cancer metastasis." Nature Med 15:59-67.



