PD Dr. Uta Höpken

Priv. Doz. Dr. Uta E. Höpken, Research Group Leader
Max-Delbrück Center for Molecular Medicine (MDC)
Robert-Rössle-Str. 10
13125 Berlin
Tel: +49 30 9406 3330
E-Mail: uhoepken(at)mdc-berlin.de
https://www.mdc-berlin.de
Charité - University Medicine Berlin
Lecturer / Privatdozentin
E-Mail: uta.hoepken(at)charite.de
Scientific Scope
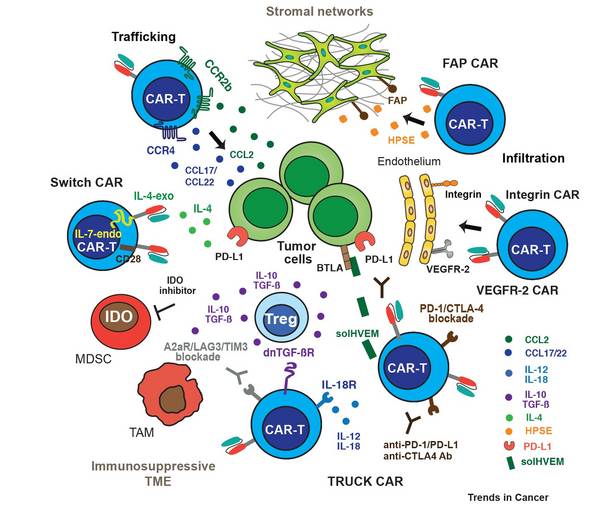
Hematological Malignancies and its Immunotherapeutic Targeting
Our group elucidates tumor-stroma interactions in hematological diseases and defines growth- and survival niches for B cell leukemia/lymphoma. By combining pre-clinical mouse models, in vivo imaging, cellular and molecular biology techniques as well as gene expression profiling substantial insight into the complex and dynamic network of tumor cells and the benign infrastructure within lymphatic tissues has been obtained. Targeting the stromal environment is an interesting therapeutic option because this benign compartment is subject to low selective pressure for genetic mutations compared with the tumor cells themselves.
Secondly, we developed Chimeric Antigen Receptor (CAR) T cells for the treatment of B cell disorders, such as a neoplastic diseases of plasma cells and mature B cells, in particular multiple myeloma (MM) or non-Hodgkin’s lymphomas (B-NHLs). Recent demonstration of disease regression of lymphoma by CD19-specific T cells has vitalized adoptive T cell therapy in the scientific community. We already succeeded to develop CAR-T cells that will allow treatment of lymphoid tumors that carry B cell differentation antigens. Examples include i) anti-tumor activity in the treatment of MM, ii) anti-plasma cell activity in dysregulated B cell responses during autoimmunity, and iii) anti-tumor activity in the treatment of mature B-NHLs.
Future Directions
During the next years, we aim to identify novel T cell antigens in different cancer entities and we will develop strategies to improve CAR T cell efficiency with the goal to overcome stromal barriers and the immunosuppressive microenvironment as well as exhaustion of therapeutic T cells. Additionally, we aim to visualize the kinetics of CAR T cell function in particular their killing capacities. For that we will employ two-photon and light sheet microscopy to quantify the spatiotemporal dynamics of CAR T cell-mediated killing in appropriate mouse models or in human 3-D cell culture systems.
Group Members
Selected References
- Bunse, M., Pfeilschifter, J., Bluhm, J., Zschummel, M., Joedicke, J. J., Wirges, A., Stark, H., Kretschmer, V., Chmielewski, M., Uckert, W., Abken, H., Westermann, J., Rehm, A., & Höpken, U. E. (2021) "CXCR5 CAR-T cells simultaneously target B cell non-Hodgkin's lymphoma and tumor-supportive follicular T helper cells." Nature communications, 12(1), 240.
- Scholz, F., Grau, M., Menzel, L., Graband, A., Zapukhlyak, M., Leutz, A., Janz, M., Lenz, G., Rehm, A., & Höpken, U. E. (2020) "The transcription factor C/EBPβ orchestrates dendritic cell maturation and functionality under homeostatic and malignant conditions." Proceedings of the National Academy of Sciences of the United States of America, 117(42), 26328–26339.
- Gloger, M., Menzel, L., Grau, M., Vion, A. C., Anagnostopoulos, I., Zapukhlyak, M., Gerlach, K., Kammertöns, T., Hehlgans, T., Zschummel, M., Lenz, G., Gerhardt, H., Höpken, U. E., & Rehm, A. (2020) "Lymphoma Angiogenesis Is Orchestrated by Noncanonical Signaling Pathways." Cancer research, 80(6), 1316–1329.
- Höpken, U. E., & Rehm, A. (2019) "Targeting the Tumor Microenvironment of Leukemia and Lymphoma." Trends in cancer, 5(6), 351–364.
- Bluhm, J., Kieback, E., Marino, S. F., Oden, F., Westermann, J., Chmielewski, M., Abken, H., Uckert, W., Höpken, U. E., & Rehm, A. (2018) "CAR T Cells with Enhanced Sensitivity to B Cell Maturation Antigen for the Targeting of B Cell Non-Hodgkin's Lymphoma and Multiple Myeloma." Molecular therapy : the journal of the American Society of Gene Therapy, 26(8), 1906–1920.
* equal contribution



