Prof. Dr. med. Clemens A. Schmitt

Cancer Genetics and Cellular Stress Responses
Charité - Universitätsmedizin Berlin
Molekulares Krebsforschungszentrum (MKFZ)
Max-Delbrück Center for Molecular Medicine (MDC) Berlin-Buch
https://www.mdc-berlin.de/schmitt#t-profile
Johannes Kepler University
Hematology/Oncology
Linz/Austria
E-Mail: clemens.schmitt(at)charite.de
Scientific Scope
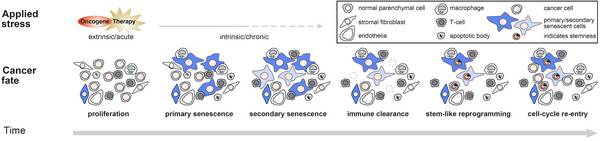
From an entity perspective, research of the lab is centered around hematological malignancies, but also reaches out to GI tract cancers, melanoma and breast cancer. We have a long-standing interest in cellular failsafe mechanisms, i.e. programmed responses to severe cellular insults, as barriers to tumor manifestation, and, likewise, as effector principles of anticancer therapies. Complementing apoptosis and autophagy, cellular senescence is the failsafe program we are mostly interested in, because this terminal cell-cycle arrest condition has far-reaching ramifications in tumor biology, immunology, and treatment outcome. We investigate molecular mechanisms and translational implications of senescence in mouse models of lymphoma and melanoma, further exploit molecular findings in patient-reminiscent clinical trial-like “panomics” mouse studies, and probe their relevance in cross-species investigations of human tumor material with annotated clinical courses of the patients the material was derived from. More specifically, test platforms for primary patient-derived material include xenografts in mice, lymphoma-on-a-chip and other novel biotechnological approaches and will implment synthetic biology, too. Our interest in senescence spans fundamental cell biology ([epi-]genomics to systems biology), dynamics/reversibility and reprogramming/plasticity (e.g. senescence-associated stemness) over time, as well as host immune interdependencies.
Future Directions
Understanding the functional implications of dynamic biological state switches for tumor behavior and patient outcome is the pivotal prerequisite of conceptually novel and more efficient treatment strategies in future cancer precision medicine in the clinic. Therefore, we apply computational biology/bioinformatics-supported multi-omics techniques (including proteomics and metabolomics) to model systems (PDX and transgenic mouse models, 3D cultures, bio chips) across all scales (tissue to single-cell level to subcellular compartments) to dissect (epi)genetic mechanisms of senescence control, to fate-track “post-senescent” cells in cancer and treatment failure, to decipher the senescence/immune cell synapse, and to exploit the anti-cancer potential of selectively senescent cells-eliminating “senolytic” therapies. Moreover, we are increasingly interested in biophysical properties of and mechanotransduction in cancer and their link to senescence. Specific PhD projects ideas may relate to these topics but do not necessarily have to – concrete PhD projects in our lab projects always evolve from personal discussions with interested PhD candidates.
Selected References
- Lee, S., Yu, Y., Trimpert, J., Benthani, F., Mairhofer, M., Richter-Pechanska, P., Wyler, E., Belenki, D., Kaltenbrunner, S., Pammer, M., Kausche, L., Firsching, T.C., Dietert, K., Schotsaert, M., Martínez-Romero, C., Singh, G., Kunz, S., Niemeyer, D., Ghanem, R., Salzer, H.J.F., Paar, C., Mülleder, M., Uccellini, M., Michaelis, E.G., Khan, A., Lau, A., Schönlein, M., Habringer, A., Tomasits, J., Adler, J.M., Kimeswenger, S., Gruber, A.D., Hoetzenecker, W., Steinkellner, H., Purfürst, B., Motz, R., Di Pierro, F., Lamprecht, B., Osterrieder, N., Landthaler, M., Drosten, C., García-Sastre, A., Langer, R., Ralser, M., Eils, R., Reimann, M., Fan, D.N.Y., Schmitt, C. A. (2021) "Virus-induced senescence is driver and therapeutic target in COVID-19." Nature 599:283-28.
- Mairhofer, M., Kausche, L., Kaltenbrunner, S., Ghanem, R., Stegemann, M., Klein, K., Pammer, M., Rauscher, I., Salzer, H.J.F., Doppler, S., Habringer, A., Paar, C., Kimeswenger, S., Hoetzenecker, W., Lamprecht, B., Lee ,S., Schmitt, C. A. (2021) "Humoral and cellular immune responses in SARS-CoV-2 mRNA-vaccinated patients with cancer." Cancer Cell 39:1171-1172.
- Milanovic, M., Fan, D.N.Y., Belenki, D., Däbritz, J.H.M., Zhao, Z., Yu, Y., Dörr, J.R., Dimitrova, L., Lenze, D., Monteiro Barbosa, I.A., Mendoza-Parra, M.A., Saleem, M.A.M., Kanashova, T., Metzner, M., Pardon, K., Reimann, M., Trumpp, A., Dörken, B., Zuber, J., Gronemeyer, H., Hummel, M., Dittmar, G., Lee, S., Schmitt, C. A. (2018) "Senescence-associated reprogramming promotes cancer stemness." Nature 553:96-100.
- Dörr, J.R., Yu, Y., Milanovic, M., Beuster, G., Zasada, C., Däbritz, J.H., Lisec, J., Lenze, D., Gerhardt, A., Schleicher, K., Kratzat, S., Purfürst, B., Walenta, S., Mueller-Klieser, W., Gräler, M., Hummel, M., Keller, U., Buck, A.K., Dörken, B., Willmitzer, L., Reimann, M., Kempa, S., Lee ,S., Schmitt, C. A. (2013) "Synthetic lethal metabolic targeting of cellular senescence in cancer therapy." Nature 501:421-425.
- Braig, M., Lee, S., Loddenkemper, C., Rudolph, C., Peters, A.H.F.M., Schlegelberger, B., Stein, H., Dörken, B., Jenuwein, T., Schmitt, C. A. (2005) "Oncogene-induced senescence as an initial barrier in lymphoma development." Nature 436:660-665.



